Functional state of adrenal cortex in junior hockey players during puberty
Фотографии:
ˑ:
Dr.Biol., Professor M.V. Shaykhelislamova1
Dr.Biol., Professor F.G. Sitdikov1
Associate Professor, PhD N.B. Dikopol'skaya1
Associate Professor, PhD G.A. Bilalova1
PhD, Dr.Hab., Professor F.R. Zotova2
1Institute of fundamental medicine and biology of Kazan (Volga region) Federal University, Kazan
2Volga Region State Academy of Physical Culture, Sport and Tourism, Kazan
Keywords: adrenal cortex, puberty, hockey players aged 11-15 years.
Introduction. The adrenal cortex (AC) plays a key role in the humoral regulation of the body’s muscular activity [1, 8, 10]. Glucocorticoids provide urgent adaptive reactions to evolve into the full development of long-term adaptation, mobilize the body’s plastic functions and prevent excessive tissue reactions to stress by means of the temporary regulatory suppression of hormone synthesis [2]. During the assessment of the glucocorticoid function of AC, it is separate studies of the levels of free and bound cortisol that are of particular importance. The transcortin-glucocorticoid complex possesses no hormonal activity, provides the transportation of glucocorticoids to tissues and is a rapidly mobilized reserve [13]. The AC androgens, that have a protein anabolic action, play a key role during the recovery period after muscular load [1]. In addition, they may serve as a defense mechanism reducing high levels of glucocorticoids and the risk of their catabolic impact on the body. Most researches carried out among athletes prove that the secretory activity of AC is considered only as an indicator of athletes’ physical fitness [7, 9], moreover, puberty hormonal changes accompanied by the physiological hyperactivity of the hypothalamic-pituitary-adrenal system are not taken into account. Excessive physical loads against the background of the functional immaturity of the system “pituitary gland-adrenal cortex” may increase the risk of development of age-related evolutive processes into the endocrine puberty dysfunctions, which is of particular importance in view of the extensive development of children and youth sports.
Objective of the research was to study the puberty changes in AC and its adaptive responses to increased physical loads in hockey players of 11-15 years of age.
Methods and structure of the study. Subject to study were 42 male athletes who were continuously observed for 5 years - from 11 through 15 years old. The children were trained in the specialized sport classes (SC) at school №1 in Kazan and practiced ice hockey. The weekly volume of physical loads amounted from 9 hours (at the age of 11) to 18 hours (at the age of 15). The examination was carried out during the competitive period and was combined with daily workouts. The 11-year-old children belonged to the initial training group of the third year of study (ITG-3); 15-year-old adolescents - to the training group of the fourth year of study (TG-4). For more reliable estimation of the specific effects of physical loads on the functional state of AC, we simultaneously examined 35 boys from the control class (CC), who attended physical education lessons at comprehensive school.
The free cortisol level (FCL) was measured based on the immune-enzymatic colorimetric method [12] with the use of the laboratory setup URINARY «FREE» CORTISOL ELISA (EIA-2989) (Germany). The bound cortisol level (BCL) was determined by the method of chemiluminescent microparticle immunoassay [11] using the optical system ARCHITECT (USA). The level of 17-ketosteroids (17-KS) was determined using the colorimetric method by N.V. Samosudova and J.J. Bass, based on the Zimmerman reaction with m-dinitrobenzene modified by M.A. Krekhova [4]. The optical density of the solution was measured using the photoelectric colorimeter FEC-56 PM (Russia).
The puberty stages (SP) were determined by the method of J. Tanner (1968), depending on the degree of manifestation of the secondary sexual characteristics [6]. The data obtained were processed by the standard variation statistics method using the software package Microsoft Excel 2007. The significance of differences was determined using the t-test, based on the Student's t-criterion.
Results and discussion. Considering that the development of endocrine glands during puberty is mainly determined by the level of sexual maturity, the study of the functional state of AC was conducted at each stage of puberty of hockey players. However, it should be noted that the distribution of boys between the sport (SC) and control classes (CC) depending on the stages of puberty had its unique features (Fig. 1).
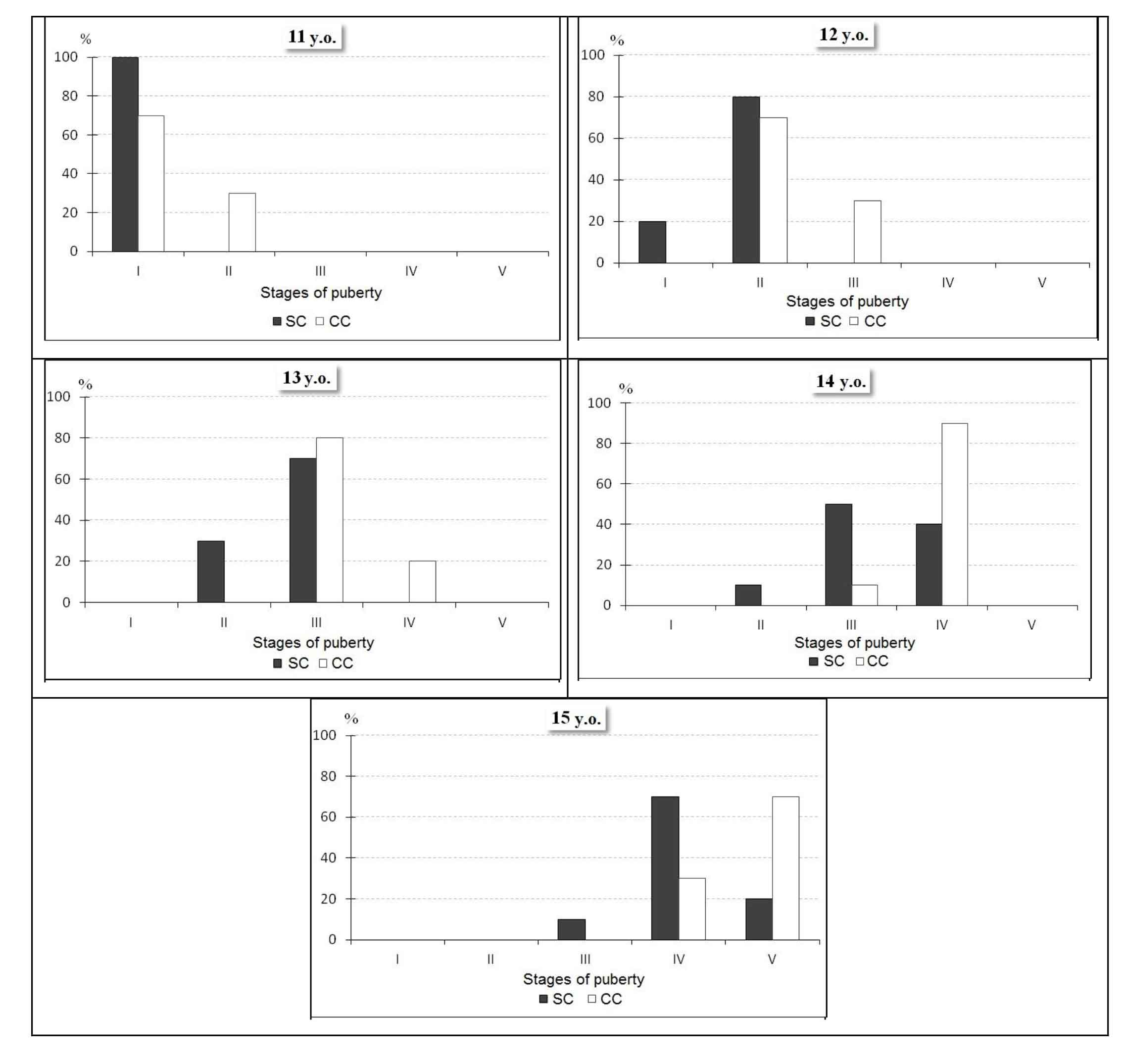
Fig. 1. Distribution of boys of 11, 12, 13, 14, 15 years of age from the sport and control classes depending on puberty stages
Thus, among the 11-year-old students not doing sports, 30% were already at the II PS, while in the group of athletes all children belonged only to the I stage. At the ages of 12 to 13 the number of boys with the III PS increased by 50% in the CC, at the same time, the II stage was not detected, and 20% of those surveyed were already at the IV stage – the stage of maximum steroidogenesis, whereas in the group of athletes 30% of children were still at the II SP, and all the rest – at the III stage. A similar trend was observed at the age of 14 and 15: if in CC 90% of 14-year-old boys were at the IV PS, 50% of athletes were only at the III stage, and 10% of athletes were still at the II stage. By the age of 15, the vast majority of the control class pupils (70%) had already entered the V PS - the stage of the final formation of the secondary sexual characteristics, and in the group of athletes it was the IV PS that prevailed, moreover, 10% of boys remained at the III stage. Therefore, junior hockey players of 11-15 years of age are characterized by a relatively slow puberty process, probably, due to increased physical loads accompanied by the glucocorticoid release in large amounts [7]. According to the literature data [3], the stable hypersecretion of glucocorticoids may affect the processes of sexual differentiation and slow down the normal functioning of the gonads. In addition, the adrenal androgens, released along with the glucocorticoids during physical exercise, can suppress the gonadoliberin secretion by the hypothalamus and the gonadotropin secretion by the pituitary gland [2].
As seen from the analysis of the peculiarities of excretion of the studied hormones and hormonal metabolites at each puberty stage (Fig. 2), the free cortisol secretion in athletes at the I-IV stages was consistently high - from 197.69±11.06 nmol/day to 227.14±14.89 nmol/day, which was significantly higher than in the control class, where these indices did not exceed 150.82±7.02 nmol/day. The cortisol secretion from the I through the II PS does not change significantly, at the III noteworthy that a simultaneous and significant decrease in the free cortisol and cortisol excretion observed from the IV through the V PS, equaling 71.58 nmol/day (p<0.05) and 16.27 mcg/day (p<0.05), respectively. The output of androgen metabolites in male athletes is oscillatory at the first peak of the 17-KS excretion as early as at the I PS, equaling 9.03±0.80 mcg/day, which is higher by 4.22 mcg/day compared to the II PS (p<0.05) and by 3.98 mcg/day compared to the CC (p<0.05). The rise in the 17-KS excretion at the I PS, which coincides with the initial phase of intensive training of hockey players, may indicate the probable involvement of androgens in the regulation of muscular activity, which is consistent with other studies in this area [1]. From the II through III SP, androgens increased by 3.04 mcg/day (p<0.05), and at the IV stage – to the utmost by 6.45 mcg/day (p<0.05). However, at the VSP the metabolite level of sex hormones decreased dramatically by 8.55 mcg/day (p<0.05). The dynamics was similar in the free cortisol and cortisol excretion. Therefore, the puberty transformations of glucocorticoid and androgen functions of AC in junior hockey players stop at the V stage of puberty. The rapid formation of the pituitary-adrenal cortex system in the children engaged in sports is observed in other studies, too, which is recognized as the training effect of systematic muscular exercises [7].
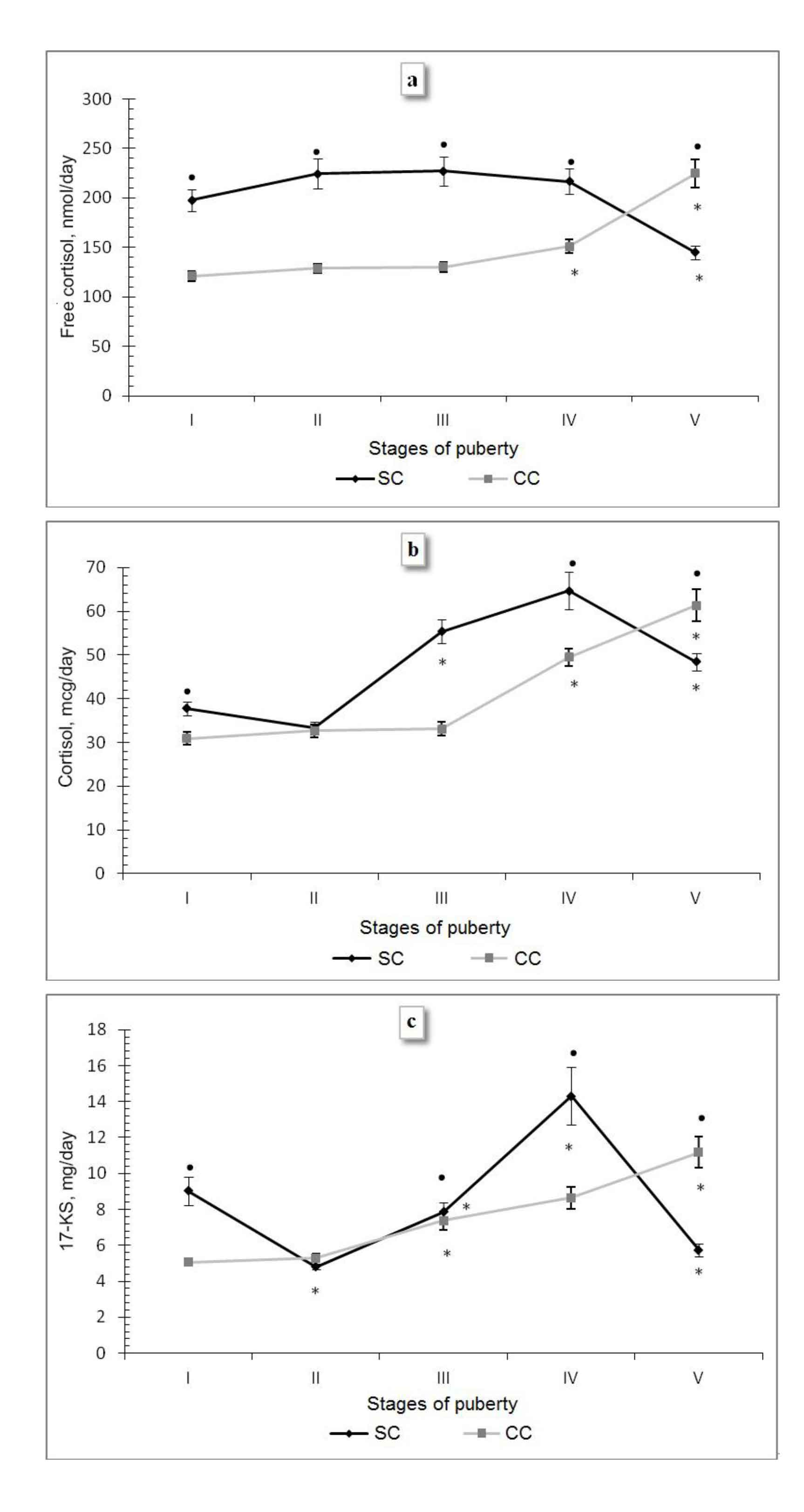
Fig. 2. Indicators of excretion of free cortisol (a), bound cortisol (b) and 17-ketosteroids (c) in the boys from the sport (SC) and control classes (CC) at different puberty stages
Note. * – the differences are significant compared with the previous puberty stage at p<0.05; • – the differences are significant between SC and CC p<0.05.
A different situation is observed among the boys from the CC – at the I, II and III PS the indicators of free cortisol and cortisol excretion remain unchanged, and in between the II and III PS the first significant increase in the metabolites of sex hormones is observed (2.09 mcg/day (p<0.05). At the IV PS, which is characterized by the intensive formation of both the adrenal glands and gonads, there is a simultaneous increase in the 17-KS excretion by 1.65 mg/day, free cortisol - by 20.67 nmol/day (p<0.05) and cortisol - by 16.46 mcg/day (p<0.05) followed by their progressive increase by the V PS by 3.34 mcg/day (p<0.05), 73.78 nmol/day (p<0.05) and 11.87 mcg/day (p<0.05), respectively. This may indicate the incomplete puberty formation of the pituitary-adrenal cortex system in the boys from the CC, which is consistent with the literature data on the later puberty changes in the regulation of pituitary-adrenocortical system, when the cortisol and dehydroepiandrosterone levels stabilize only by the age 21 [5].
Conclusions. Physical loads in the form of regular exercises are the dominant factor in the development of the AC functions and puberty of adolescents. Consistently high levels of excretion of free cortisol in junior hockey players up to the IV stage of puberty, which significantly exceed similar values in the boys from the CC, indicate the stress effects of increased physical loads, especially at the initial stages of the training process. At the same time, the rising level of free cortisol coincides with the fluctuating cortisol excretion, which testifies to the gradual formation of glucocorticoid reserve in the process of training and puberty of athletes.
As a result of specific effects of sport loads on the child's body, there is a relative slowdown of the process of sexual maturation of junior hockey players (development of the secondary sexual characteristics), which can be explained in terms of the inhibitory effect of high glucocorticoid level in the process of sexual differentiation and gonad functioning.
References
- Viru A.L. Gormony i sportivnaya rabotosposobnost' (Hormones and physical work capacity) / A.L. Viru, P.K. Kyirge. – Moscow: Fizkul'tura i sport, 1983.
- Drzhevetskaya I.A. Endokrinnaya sistema rastushchego organizma (Endocrine system of growing body) / I.A. Drzhevetskaya. – Moscow: Vysshaya shkola, 1987.
- Katsiya G.V. Narushenie balansa steroidnykh gormonov pri ozhirenii i metabolicheskom sindrome u muzhchin molozhe 40 let (Steroid hormone imbalance in obesity and metabolic syndrome in men under 40 years old) / G.V. Katsiya, N.P. Goncharov, N.A. Chagina // Problemy endokrinologii. – 2011. – V. 57. – № 4. – P. 7-12.
- Kolb V.G. Klinicheskaya biokhimiya (Clinical biochemistry) / V.G. Kolb, V.S. Kamyshnikov. – Minsk, 1976.
- Selverova N.B. Fiziologiya razvitiya rebenka (Child Development Physiology) / N.B. Selverova. – Moscow: Obrazovanie ot A do Ya, 2000. – P. 104-126.
- Tanner J. Biologiya cheloveka (Human Biology) / J. Tanner. – Moscow, 1968. – P. 247-326.
- Fomin N.A. Fiziologiya cheloveka (Human Physiology) / N.A. Fomin. – Moscow: Prosveshchenie, 2003. – 3rd ed.
- Shaykhelislamova M.V. Gormonal'ny status i vegetativny sindrom u detey 7-15-letnego vozrasta (Hormonal status and vegetative syndrome in 7-15 year olds) / M.V. Shaykhelislamova, F.G. Sitdikov, A.A. Sitdikova et al. // Byul. eksper. biol. (Bulletin of Experimental Biology and Medicine). – 2012. – V. 154. – № 12. – P. 677-681.
- Arbay O.C., Senocak M.E., Cahit T.F. // J.Pediatr.Surg. – 2001. – Vol. 36. – N. 4. – P. 549-554.
- Chrousos G.P. // Endocr. Res. – 2000. – Vol. 26. – N. 4. – P. 513-514.
- Collins W.P., Barnard G.J., Kim J.B. et al. // Immunoassays for Clinical Chemistry. Edinburgh: Churchill livingstone, 1983. – P. 373-397.
- Davidsohn I., Henry J.B. Clinical diagnosis and management by laboratory methods. Philadelphia. PA: W.B.Saunders, 1979. – P. 9-408.
- Ostrander M.M., Ulrich-Lai Y.M., Choi D.C. // Ibid. – 2006. – Vol. 147. – N. 4. – P. 2008-2017.




 Журнал "THEORY AND PRACTICE
Журнал "THEORY AND PRACTICE